Determine Which Ions Are Present in Each of the Compounds
C12- O Cr3 O 02- O Cr O 03- 00- I crt Pb 3 00000 C14- Pb4 Na PO Al C10. If youre seeing this message it means were having trouble loading external resources on our website.

Question Video Identifying The Ions Present In The Ionic Compound Ammonium Chloride Nagwa
Determine which ions are present in cach of the compounds C0 Na PO ANCIO Question Transcribed Image Text.

. Up to 256 cash back Get the detailed answer. LIMITED TIME OFFER. GET 20 OFF GRADE YEARLY SUBSCRIPTION.
Solution for Determine which ions are present in each of the compounds. To determine the charge of each of these components take a look at the subscript. Cr РЬСІ 0- Pb 0 ОРЬСІ Cr2 CI Crt C14- 02- Pb C12- Cr Na PO AlCIO CI p3 02- PO Nat OCIO Al Question.
It means the charge of Na is 1 not written while the charge of PO4 is 3. A SiF 4 and LaF 3 b FeCl 2 and ReCl 6 c PbCl 4 and RbCl. First we need to get the mass of MgI2 to moles of MgI2 we do this using the formula weight of MgI2 2781 grams mole in order for our units to cancel we need to conver the mg to g 35 mg 00035 grams.
Lets practice identifying the ions present in a given ionic compound by knowing the number of electrons it gains or loses to become stable form octet. Use the appropriate naming convention for ionic or molecular substances to assign a name to each compound. Li and O2-.
A FeCl 2 b HNO 3 c NH 4 2 SO 4 d CaOH 2. Na and PO43-AlClO43. Find step-by-step Chemistry solutions and your answer to the following textbook question.
Determine the oxidation numbers of all the elements in each of the following compounds. The number of ions in a compound depends on the structure of the compound and the oxidation states of the elements within the compound. Li2O Mg2 and S2-.
Specify what ions are present in solution upon dissolving each of the Specify what ions are present in solution upon dissolving each of the following substances in water. Determine which ions are present in each of the compounds. PO cio CINE CI 7 02- Al Nap2 A13 M Na O co- Srso Sr Sr2 Sp OOOOOO so so- so-.
AlCl3 Na and N3-. Cr og PbCI PbCl. Cr3 and O2-PbCl4.
Determine which ions are present in each of the compounds. Determine which ions are present in each of the compounds. Determine which ions are present in each of the compounds.
Determine the formula unit for the compound formed when each pair of ions interact. Determine which ions are present in cach of the compounds. Up to 24 cash back 757 Determine whether each compound is soluble or insoluble.
00035 grams x 1 mole 2781 grams moles of MgI2. Look at the ions present a. ОРЬ ОРЫ Na PO4 Al CIO 0 02 02- Nap2 Nat CIO Co ОСІ O Art PO p3 PO- O A13 SrSOA Sr SO Sr2 Name the compounds.
How to Find the Number of Ions in a Compound. For each soluble compound identify the ions present in. Determine what ions are present in the following compounds.
The subscript represents the charge of the other element. Up to 256 cash back In the following pairs of binary compounds determine which one is a molecular substance and which one is an ionic substance. Hence the ions are Pb 4 and Cl-Item 3 Na 3 PO 4 Here the compound is composed of Na and polyatomic compound PO4.
An elements oxidation state is the number of electrons that an atom possesses or lacks relative to the number of protons in its nucleus. Cr0 РЬСІ Осі Crt Осне Do-. CrO P6CI Cr C14- Cr³ Pb4 Pb 3 Cl- 02- O PBCI Cr Incorrect Incorrect NaPO4.
Determine whether each compound is soluble or insoluble. Determine what ions are present in the following compounds. MgS Al3 and Cl-.
Determine which ions are present in each of the compounds. How many magnesium ions are present in 35 mg of magnesium iodide. Оо- Ос ОСТ ОРЬСІ.
Pb4 and Cl-Na3PO4. If the compound is soluble list the ions present in solution.
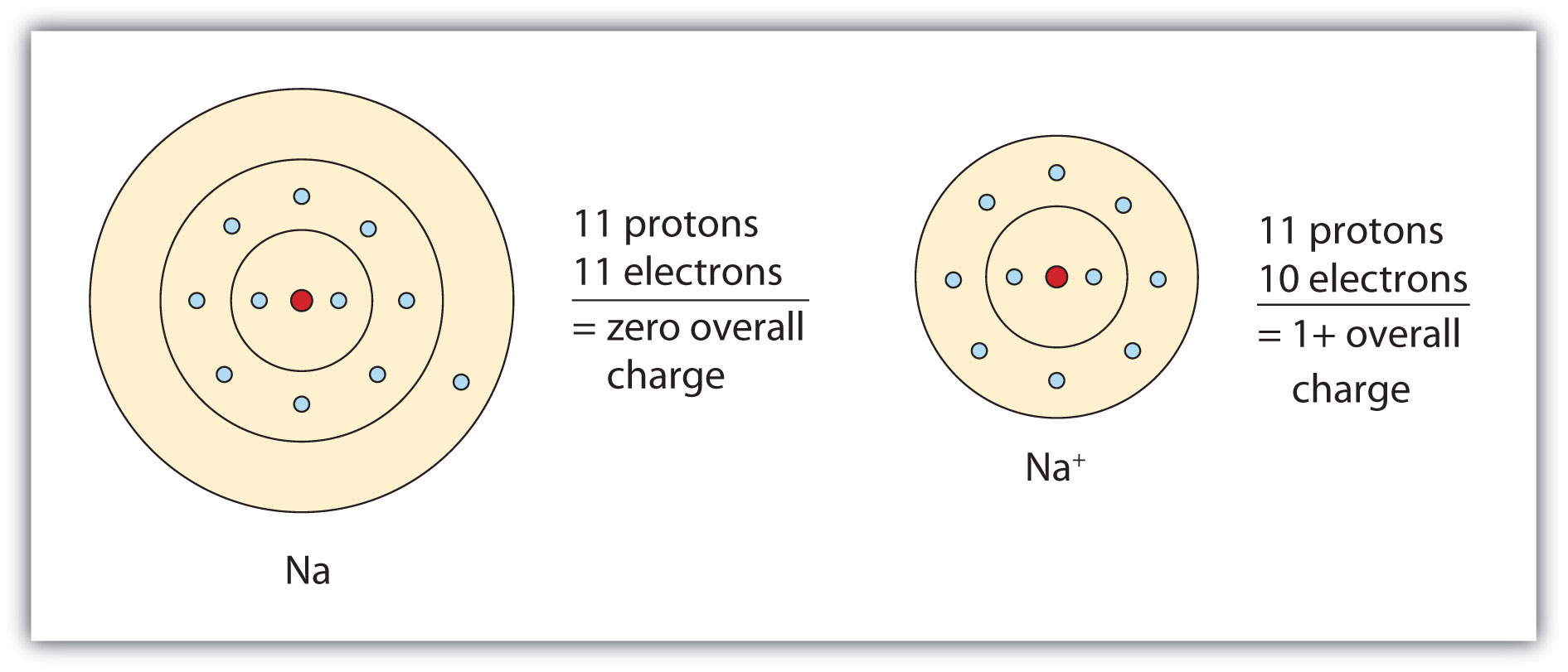
Ch103 Chapter 4 Ions And Ionic Compounds Chemistry
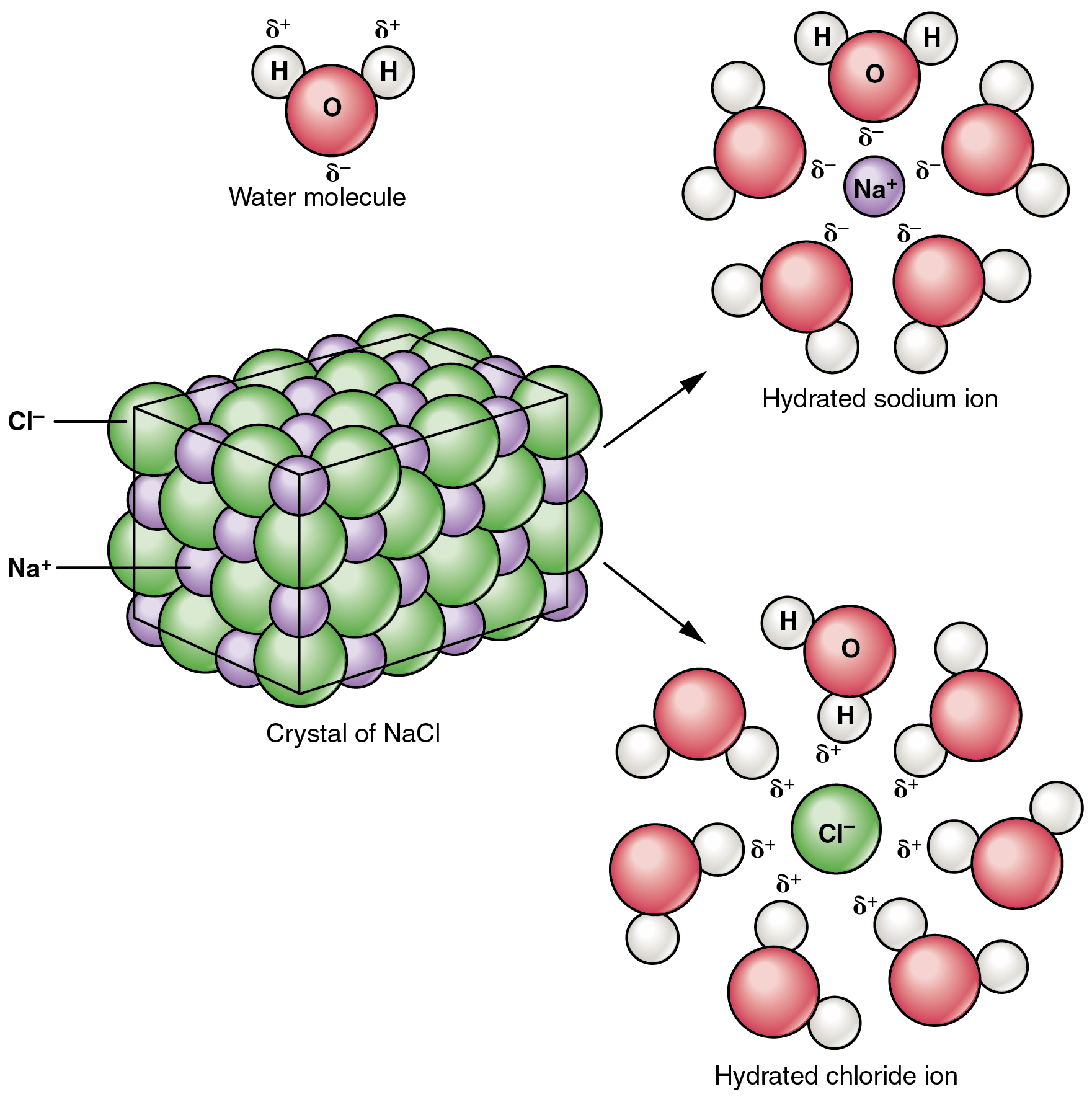
Molecular Complete Ionic And Net Ionic Equations Article Khan Academy

Question Video Identifying The Ions Present In A Solution Of Strontium Chloride Nagwa
Comments
Post a Comment